ESR blog
High ambient temperatures affect animal production and welfare in tropical and sub-tropical regions of the world (He et al., 2018). Global climate change is primarily caused by greenhouse gas (GHG). The livestock sector contributes 14.5% of global GHG emissions (Gerber et al., 2013). The effects of climate change such as heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… affects livestock production and eventually causing diseases and reducing the production performance of farm animals (Friel et al., 2009). The poultry sector is also face with the impact of climatic change coupled with global warming (Chang et al. 2020; Sohail et al. 2012). Due to the variations of global climate (environmental temperatures, humidity, precipitation patterns, and raising atmospheric carbon dioxide level), the poultry industry is severely affected (Nawab et al. 2018). However, among these environmental stress factors, heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… (HS) is one of the most important factor influencing poultry performances including reduced feed intake, which in turn, affects growth rate, body weight, meat quality, egg quality, egg production, semen quality, and fertility; which cause huge financial losses in the poultry sector (Nawab et al. 2018; Kumar et al. 2021; Goel 2021). The decrease in the productive potential of birds exposed to HS may be attributed to the deviation of energy resources from production to adaptation pathway (Vandana et al. 2021).
High ambient temperature has a negative impact on the performance traits of poultry. Thus, the selection of birds for high performance has increased their susceptibility to HS (Kumar et al. 2021). According to a report by (He et al., 2018), in the US, an amount of $128-165 million is lost from the poultry industry annually due to HS effects.
Therefore, it is essential to mitigate the deleterious effects caused by HS in poultry. Several strategies have been explored to address the adverse effects of HS in poultry which include genetic marker selection to enhance thermo-tolerance and productivity of poultry birds in hot regions of the world (Nawab et al. 2018), in ovo administration of bioactive substances (Slawinska et al. 2020; Elnesr et al. 2019; Zhu et al. 2020; Goel et al. 2021), housing management (Kapetanov et al. 2015; Pawar et al. 2016), early heat conditioning (Kang et al. 2019), thermal manipulation (Ncho et al. 2021; Li et al. 2007; Goel et al. 2021; Zaboli et al. 2017) and feeding management (Lin et al. 2006; Goel 2021; Li et al. 2007).
In ovo supplementation of bioactive substances
One intervention strategy to ameliorate the adverse effects of HS is the in ovo administration of bioactive substances (Slawinska et al. 2019). PrebioticsSelectively fermented ingredients that allows specific changes in the composition or activity of the… such as galactooligosaccharide (GOS) (Slawinska et al. 2020; Tavaniello et al. 2020) have been reported to improve poultry birds thermotolerance to HS. The in ovo administartion of 3.5 mg GOS/egg on 12th embryonic day (ED) could mitigate the detrimental effect of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… on some meat quality traits. Concomitantly, several research have shown that some amino acids and vitamins delivered on 17.5 ED, 18 ED, 15 ED, in ovo respectively improved the chicks’ oxidative state, which led to improvements in incubationKeeping the eggs under defined conditions (ideally 37.8℃ and 55% relative humidity) for the chicks… results, chick quality and production performances (Ncho et al. 2021; Sgavioli et al. 2019; Zhu et al. 2020; Araujo et al. 2019), and minerals (McGruder et al. 2011). In a different study, it is reported that in ovo injection of L-Leu on 7 ED promoted the recovery of antioxidative status in broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… after exposure to HS (Han et al. 2018).
Genetic selection of poultry
There is variation between poultry breeds in their level of thermo-tolerance (Mashaly et al. 2004), making genetic modification a potential method to alleviate HS. Genetic selection is a process of selecting good quality birds to produce the next progeny. Various aspects such as growth and immunity have been used as a selective parameter. The major problem with meat-type chicken has been associated with deprived feed intake under HS (Awad et al. 2020). Continuous selection in the last few decades targeted rapid growth in broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of…. However, they are comparably more affected under HS due to lower heat tolerance compared to slow-growing broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… (Deeb and Cahaner 2002). Egg production performances are the main criteria for rearing laying hens. HS adversely reduces egg production and quality (Barrett et al. 2019). However, birds’ genetic selection helps reduce the decline in egg production performance and egg quality in laying hens (Radwan 2020). Fine-mapping with quantitative trait loci (QTL) is also effective for the screening of high HS tolerant birds. Identification of QTL for important parameters such as body temperature and weight is the essential requirement for such studies. Previous studies suggested that QTL enables the selection of heat-tolerant birds for improved growth performances in chickens (Van Goor et al. 2015). In marker-assisted selective breeding of poultry birds, polymorphisms in HSP genes, dwarf gene, frizzle gene, and naked neck genes are some of the important thermo-tolerant genes that can be used to develop a breed with superior thermotolerance (Chen et al. 2013; Yu et al. 2008).
Dietary and Nutritional Strategies
Among environmental stresses, the most significant factor influencing feed intake and subsequently weight gain in broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… is the ambient temperature. Several authors have demonstrated that bird’s growth depression because of HS can be partially alleviated by increasing the calorie content of the diet (Wasti et al., 2020; Attia et al., 2017). While the metabolismChemical processes that occur within a living organism in order to maintain life. process, fat produces less heat compared to protein and carbohydrates, resulting in lower body temperature in birds. Fats and oil not only improve the energy density of other feed components but also slow down the food passage rate resulting into increased nutrient utilization (Attia et al., 2018). Dietary supplementation with up to 5% fats or oils helps to minimize heat generation due to their higher energy content and lower heat increase compared to carbs and proteins. In heat-stressed laying hens, increasing fat in the diet by 5% was found to increase feed intake by 17% (Daghir, 2008). Similarly, a significant increase in broiler performance was seen when the 5% fat diet was given (Ghazalah et al., 2008). In addition, broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… performance, meat lipids, and physiological and immunological features were all reportedly improved by increasing the oil supplementation in diets with greater protein concentrations (Attia et al., 2017). Care should be taken when choosing fats or oils because certain of them, such as soybean oil, canola oil, walnuts, flaxseed oil, and fish oil, have greater quantities of polyunsaturated fatty acids and should be avoided or be used at minimal levels in the diet.
Besides energy, the balance of amino acids in the diet must be taken into account during HS. If the energy level of the diet is increased, the other nutrients must be increased proportionally. Lower crude protein (CP) diets supplemented with limiting amino acids (mostly methionine and lysine) perform better than high CP diets during HS. Additionally, other studies found that lower CP diets resulted with lower FI (Awad et al., 2017, 2018). In addition to a low CP diet, several studies examined the effects of a high CP diet. During HS, High CP diets led to either an increase or a decrease in BWG and a reduction in FCR (Cheng et al., 1999; Faria Filho et al. (2005). However, some studies (Laudadio et al., 2012; Soares et al., 2020) found no difference between high and conventional CP diets, and the higher expense of high CP diets could result in negative economic effects (Cardoso et al., 2022).
Vitamins are undeniably significant components of poultry diets due to their anti-stress properties and decreased synthesis in HS. Vitamins A, E, and C supplements have gotten a lot of scientific attention as an antioxidant in chickens under HS conditions, along with other vitamins. All of them are efficient in slowing down the lipid peroxidation process by removing free radical forms under high heat in cell and subcellular organ membranes (Khattak et al., 2012).
Feeding Strategies
The strategy of withdrawing the feed before six hours of peak temperature time has been found promising as the passage rate allows feed to remain in the gut for up to six hours. Withholding food for at least 6 hours under HS reduced the broilers’ body temperatures, mortality, and heterophil-to-lymphocyte ratio, reducing the harmful consequences of HS (Yalçin et al., 2001, Lozano et al., 2006). Another strategy of dual feeding has been practiced in broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… by giving the birds a protein-rich portion of diet during the cooler part of the day and an energy-rich portion of diet during the warmer phase of the day (Teyssier et al., 2022). While a dual-feeding approach might be feasible in tropical areas and less-intensive production systems. Iyasere et al. (2021) estimated that it is unsuitable for most commercial production operations due to cost and logistical constraints. The most crucial component for broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… nutrition is water, which is also crucial for thermoregulation in hot environments. During HS, wet mash feeding is also suggested since the wet feed promotes micronutrient absorption by boosting total dry matter intake. Awojobi et al. (2009) and Dei and Bumbie (2011) found increased BWG in wet-fed birds raised in tropical settings (addition of 1 to 2 parts water to 1 part dry diet).
Housing Management
The proper design of the poultry shed might contribute to decrease HS by keeping optimal conditions within the shed. The shed’s roof that is between 10 and 12 meters high, which serves to lessen the intensity of direct solar radiation (Bhadauria et al. 2016). Other strategies to reduce radiation penetration, both direct and indirect, include insulating the roof and building the roof with an overhang (Oloyo and Ojerinde 2019). Overcrowding makes it harder for birds to lose heat and promotes disease transmission. Therefore, it is essential to provide enough floor space during heat waves. When birds are under HS, it might be beneficial to reduce overcrowding, so they have appropriate access to food and water (Kapetanov et al. 2015). With technological advancements, there has recently been an upsurge in the utilization of a closed house system for more intensive farming systems (NaRanong, 2007). However, such houses are expensive to build and operate in developing nations (Glatz and Pym, 2013).
Conclusions
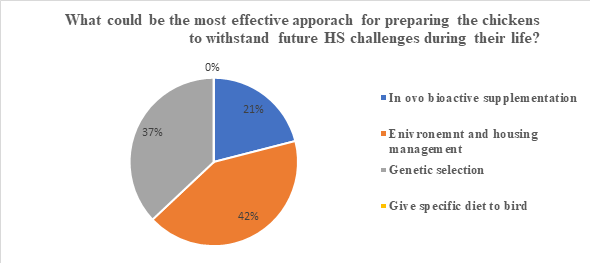
The gradual increase of temperatures, and global warming coinciding with the expansion of the poultry industry in the tropical and subtropical regions makes it important to provide novel intervention strategies to mitigate the effects of stress. The effects of HS cause gut dysbiosis subsequently weakening the immune system of broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… and making them more prone to pathogen infection. This also leads to poor production performance, mortality, and eventually leading to financial losses. Up to date, numerous strategies have been practiced, and new approaches are being tested to combat the adverse effects of HS on poultry production. Most of the approaches include in ovo administration of bioactive substances, genetic selection, dietary and feeding strategies and housing management that have proven beneficial to mitigate the detrimental effects of HS and provide aid to birds to maintain their growth. We created a poll and asked a question entitled: What could be the most effective approach for preparing the chickens to withstand future HS challenges during their life? This question with four possible answers was shared on the MonoGutHealth LinkedIn page for people to vote. After the polls, the results demonstrated that 42% of the people who voted shared that good house management is the most effective approach to mitigate HS in poultry, whereas 37% thought that genetic selection could be a potential strategy for this problem. Furthermore, in the case of in ovo supplementation of bioactive substances, only 12% suggested that it could ameliorate the adverse effects of HS in chickens, and 0% voted for specific diets for birds. In total, there were 19 votes.
In a nutshell, from the result of the votes, do you think that environment and housing management is the most effective approach for preparing chickens to withstand future heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… challenges?
References
Attia, Y.A. and Hassan, S.S., 2017. BroilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… tolerance to HS at various dietary protein/energy levels. European Poultry Science, 81(10.1399).
Attia, Y.A., Al-Harthi, M.A. and Sh. Elnaggar, A., 2018. Productive, physiological and immunological responses of two broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… strains fed different dietary regimens and exposed to HS. Italian Journal of Animal Science, 17(3), pp.686-697.
Araujo, I. C. S., M. B. Cafe, R. A. Noleto, J. M. S. Martins, C. J. Ulhoa, G. C. Guareshi, M. M. Reis, and N. S. M. Leandro. 2019. ‘Effect of vitamin E in ovo feeding to broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… embryos on hatchability, chick quality, oxidative state, and performance’, Poult Sci, 98: 3652-61.
Awad, E. A., M. Najaa, Z. A. Zulaikha, I. Zulkifli, and A. F. Soleimani. 2020. ‘Effects of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… strains’, Asian-Australas J Anim Sci, 33: 778-87.
Awad, E.A., Zulkifli, I., Soleimani, A.F. and Aljuobori, A., 2017. Effects of feeding male and female broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… on low-protein diets fortified with different dietary glycine levels under the hot and humid tropical climate. Italian Journal of Animal Science, 16(3), pp.453-461.
Awojobi, H.A., Oluwole, B.O., Adekunmisi, A.A. and Buraimo, R.A., 2009. Performance of finisher broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… fed wet mash with or without drinking water during wet season in the tropics. International Journal of Poultry Science, 8(6), pp.592-594.
Barrett, N. W., K. Rowland, C. J. Schmidt, S. J. Lamont, M. F. Rothschild, C. M. Ashwell, and M. E. Persia. 2019. ‘Effects of acute and chronic heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… on the performance, egg quality, body temperature, and blood gas parameters of laying hens’, Poult Sci, 98: 6684-92.
Cardoso, D.M., Cardeal, P.C., Soares, K.R., Sousa, L.S., Castro, F.L.S., Araújo, I.C.S. and Lara, L.J.C., 2022. Feed form and nutritional level for rearing growing broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… in thermoneutral or HS environments. Journal of Thermal Biology, 103, p.103159.
Chang, Q., Y. Lu, and R. Lan. 2020. ‘Chitosan oligosaccharide as an effective feed additive to maintain growth performance, meat quality, muscle glycolytic metabolismChemical processes that occur within a living organism in order to maintain life. , and oxidative status in yellow-feather broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… under heat stress’, Poult Sci, 99: 4824-31.
Cheng, T.K., Hamre, M.L. and Coon, C.N., 1999. Effect of constant and cyclic environmental temperatures, dietary protein, and amino acid levels on broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… performance. Journal of Applied Poultry Research, 8(4), pp.426-439.
Chen, Z. Y., J. K. Gan, X. Xiao, L. Y. Jiang, X. Q. Zhang, and Q. B. Luo. 2013. ‘The association of SNPs in Hsp90beta gene 5′ flanking region with thermo tolerance traits and tissue mRNA expression in two chicken breeds’, Mol Biol Rep, 40: 5295-306.
Daghir, N.J. ed., 2008. Poultry production in hot climates. Cabi.
Deeb, N., and A. Cahaner. 2002. ‘Genotype-by-environment interaction with broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… genotypes differing in growth rate. 3. Growth rate and water consumption of broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… progeny from weight-selected versus nonselected parents under normal and high ambient temperatures’, Poult Sci, 81: 293-301.
Dei, H. K., and Bumbie, G. Z. (2011). Effect of wet feeding on growth performance of broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… in a hot climate. Br. Poult. Sci. 52, 82–85.
Elnesr, S. S., H. A. M. Elwan, Q. Q. Xu, C. Xie, X. Y. Dong, and X. T. Zou. 2019. ‘Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… chicks exposed to heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… during incubation’, Poult Sci, 98: 2290-98.
Faria Filho, D.E., Rosa, P.S., Vieira, B.S., Macari, M. and Furlan, R.L., 2005. Protein levels and environmental temperature effects on carcass characteristics, performance, and nitrogen excretion of broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… from 7 to 21 days of age. Brazilian Journal of Poultry Science, 7, pp.247-253.
Friel, S., Dangour, A. D., Garnett, T., Lock, K., Chalabi, Z., Roberts, I., Butler, A., Butler, C. D., Waage, J., McMichael, A. J., & Haines, A. (2009). Public health benefits of strategies to reduce greenhouse-gas emissions: Food and agriculture. The Lancet, 374(9706), 2016–2025. https://doi.org/10.1016/S0140-6736(09)61753-0
Gerber, P. J., Hristov, A. N., Henderson, B., Makkar, H., Oh, J., Lee, C., Meinen, R., Montes, F., Ott, T., Firkins, J., Rotz, A., Dell, C., Adesogan, A. T., Yang, W. Z., Tricarico, J. M., Kebreab, E., Waghorn, G., Dijkstra, J., & Oosting, S. (2013). Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal, 7, 220–234. https://doi.org/10.1017/S1751731113000876
https://doi.org/10.1017/S0043933918000727
Ghazalah, A.A., Abd-Elsamee, M.O. and Ali, A.M., 2008. Influence of dietary energy and poultry fat on the response of broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… chicks to heat therm. Int. J. Poult. Sci, 7(4), pp.355-359.
Glatz, P. and Pym, R., 2013. Poultry housing and management in developing countries. Poultry Development Review; FAO: Rome, Italy, pp.24-28.
Goel, A. 2021. ‘Heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… management in poultry’, J Anim Physiol Anim Nutr (Berl), 105: 1136-45.
Goel, A., C. M. Ncho, C. M. Jeong, and Y. H. Choi. 2021. ‘Embryonic Thermal Manipulation and in ovo Gamma-Aminobutyric Acid Supplementation Regulating the Chick Weight and Stress-Related Genes at Hatch’, Front Vet Sci, 8: 807450.
Han, G., H. Yang, T. Bungo, H. Ikeda, Y. Wang, L. T. N. Nguyen, H. M. Eltahan, M. Furuse, and V. S. Chowdhury. 2018. ‘In ovoL-leucine administration stimulates lipid metabolisms in heat-exposed male, but not female, chicks to afford thermotolerance’, J Therm Biol, 71: 74-82.
He, S. P., Arowolo, M. A., Medrano, R. F., Li, S., Yu, Q. F., Chen, J. Y., & He, J. H. (2018). Impact of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… and nutritional interventions on poultry production. World’s Poultry Science Journal, 74(4), 647–664.
Iyasere, O.S., Bateson, M., Beard, A.P. and Guy, J.H., 2021. Provision of Additional Cup Drinkers Mildly Alleviated Moderate HS Conditions in Broiler ChickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of…. Journal of Applied Animal Welfare Science, 24(2), pp.188-199.
Kang, D., J. Park, and K. Shim. 2019. ‘Heat Treatment at an Early Age Has Effects on the Resistance to Chronic Heat StressA condition when animals do not have the ability to withstand high ambient temperatures especially w… on Broilers’, Animals (Basel), 9.
Kapetanov, Miloš, Marko Pajić, Dragana Ljubojević, and Miloš Pelić. 2015. ‘Heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… in poultry industry’, Archives of Veterinary Medicine, 8: 87-101.
Khattak, F.M., Acamovic, T., Sparks, N., Pasha, T.N., Joiya, M.H., Hayat, Z. and Ali, Z., 2012. Comparative efficacy of different supplements used to reduce HS in broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of…. Pakistan Journal of Zoology, 44(1).
Kumar, M., P. Ratwan, S. P. Dahiya, and A. K. Nehra. 2021. ‘Climate change and heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w…: Impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies’, J Therm Biol, 97: 102867.
Laudadio, V., Dambrosio, A., Normanno, G., Khan, R.U., Naz, S., Rowghani, E. and Tufarelli, V., 2012. Effect of reducing dietary protein level on performance responses and some microbiological aspects of broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… under summer environmental conditions. Avian Biology Research, 5(2), pp.88-92.
Li, X. J., X. S. Piao, S. W. Kim, P. Liu, L. Wang, Y. B. Shen, S. C. Jung, and H. S. Lee. 2007. ‘Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… chickens’, Poult Sci, 86: 1107-14.
Lin, H, HC Jiao, Johan Buyse, and Eddy Decuypere. 2006. ‘Strategies for preventing heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… in poultry’, World’s Poultry Science Journal, 62: 71-86.
Lozano, C., De Basilio, V., Oliveros, I., Alvarez, R., Colina, I., Bastianelli, D., Yahav, S. and Picard, M., 2006. Is sequential feeding a suitable technique to compensate for the negative effects of a tropical climate in finishing broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of…?. Animal Research, 55(1), pp.71-76.
Mashaly, M. M., G. L. Hendricks, 3rd, M. A. Kalama, A. E. Gehad, A. O. Abbas, and P. H. Patterson. 2004. ‘Effect of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… on production parameters and immune responses of commercial laying hens’, Poult Sci, 83: 889-94.
McGruder, B. M., W. Zhai, M. M. Keralapurath, L. W. Bennett, P. D. Gerard, and E. D. Peebles. 2011. ‘Effects of in ovo injection of electrolyte solutions on the pre- and posthatch physiological characteristics of broilers’, Poult Sci, 90: 1058-66.
Musharaf, N.A. and Latshaw, J.D., 1999. Heat increment as affected by protein and amino acid nutrition. World’s Poultry Science Journal, 55(3), pp.233-240.
Nawab, A., F. Ibtisham, G. Li, B. Kieser, J. Wu, W. Liu, Y. Zhao, Y. Nawab, K. Li, M. Xiao, and L. An. 2018. ‘Heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry’, J Therm Biol, 78: 131-39.
NaRanong, V., 2007. Structural changes in Thailand’s poultry sector and its social implications. Thailand Development Research Institute. Bangkok, Thailand.
Ncho, C. M., A. Goel, C. M. Jeong, M. Youssouf, and Y. H. Choi. 2021. ‘In Ovo Injection of GABA Can Help Body Weight Gain at Hatch, Increase Chick Weight to Egg Weight Ratio, and Improve BroilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… Heat Resistance’, Animals (Basel), 11.
Pawar, SS, B Sajjanar, VD Lonkar, NP Kurade, AS Kadam, AV Nirmal, MP Brahmane, and SK Bal. 2016. ‘Assessing and mitigating the impact of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… in poultry’, Adv. Anim. Vet. Sci, 4: 332-41.
Radwan, L. M. 2020. ‘Genetic improvement of egg laying traits in Fayoumi chickens bred under conditions of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… through selection and gene expressionA process by which information from a gene is used for protein synthesis. Ultimately this whole proc… studies’, J Therm Biol, 89: 102546.
Sgavioli, S., V. R. De Almeida, J. B. Matos Junior, G. L. Zanirato, L. L. Borges, and I. C. Boleli. 2019. ‘In ovo injection of ascorbic acid and higher incubationKeeping the eggs under defined conditions (ideally 37.8℃ and 55% relative humidity) for the chicks… temperature modulate blood parameters in response to heat exposure in broilers’, Br Poult Sci, 60: 279-87.
Slawinska, A., S. Mendes, A. Dunislawska, M. Siwek, M. Zampiga, F. Sirri, A. Meluzzi, S. Tavaniello, and G. Maiorano. 2019. ‘Avian model to mitigate gut-derived immune response and oxidative stress during heat’, Biosystems, 178: 10-15.
Slawinska, A., M. Zampiga, F. Sirri, A. Meluzzi, M. Bertocchi, S. Tavaniello, and G. Maiorano. 2020. ‘Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… on performance and welfare of broilers’, Poult Sci, 99: 407-15.
Sohail, M. U., M. E. Hume, J. A. Byrd, D. J. Nisbet, A. Ijaz, A. Sohail, M. Z. Shabbir, and H. Rehman. 2012. ‘Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilersChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… subjected to chronic heat stress’, Poult Sci, 91: 2235-40.
Sun, X., Zhang, H., Sheikhahmadi, A., Wang, Y., Jiao, H., Lin, H. and Song, Z., 2015. Effects of HS on the gene expressionA process by which information from a gene is used for protein synthesis. Ultimately this whole proc… of nutrient transporters in the jejunum of broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… (Gallus gallus domesticus). International journal of biometeorology, 59(2), pp.127-135.
Tavaniello, S., A. Slawinska, D. Prioriello, V. Petrecca, M. Bertocchi, M. Zampiga, G. Salvatori, and G. Maiorano. 2020. ‘Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… exposed to heat stress’, Poult Sci, 99: 612-19.
Thornton, Philip K, Randall B Boone, and Julián Ramírez Villegas. 2015. ‘Climate change impacts on livestock’, CCAFS Working Paper.
Teyssier, J.R., Brugaletta, G., Sirri, F., Dridi, S. and Rochell, S.J., 2022. A review of HS in chickens. Part II: Insights into protein and energy utilization and feeding. Frontiers in Physiology, p.1521.
Van Goor, A., K. J. Bolek, C. M. Ashwell, M. E. Persia, M. F. Rothschild, C. J. Schmidt, and S. J. Lamont. 2015. ‘Identification of quantitative trait loci for body temperature, body weight, breast yield, and digestibility in an advanced intercross line of chickens under heat stress’, Genet Sel Evol, 47: 96.
Vandana, G. D., V. Sejian, A. M. Lees, P. Pragna, M. V. Silpa, and S. K. Maloney. 2021. ‘Heat stressA condition when animals do not have the ability to withstand high ambient temperatures especially w… and poultry production: impact and amelioration’, Int J Biometeorol, 65: 163-79.
Yalcin, S., Özkan, S., Türkmut, L. and Siegel, P.B., 2001. Responses to HS in commercial and local broilerChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… stocks. 1. Performance traits. British Poultry Science, 42(2), pp.149-152.
Yu, J., E. Bao, J. Yan, and L. Lei. 2008. ‘Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers’, Cell Stress Chaperones, 13: 327-35.
Zaboli, G. R., S. Rahimi, F. Shariatmadari, M. A. Torshizi, A. Baghbanzadeh, and M. Mehri. 2017. ‘Thermal manipulation during Pre and Post-Hatch on thermotolerance of male broiler chickensChickens kept for meat production. Fast growing breeds can reach a weight of over 2 kg at 5 weeks of… exposed to chronic heat stress’, Poult Sci, 96: 478-85.
Zhu, Y. F., M. B. Bodinga, J. H. Zhou, L. Q. Zhu, Y. L. Cao, Z. Z. Ren, and X. J. Yang. 2020. ‘Effects of in ovo injection of vitamin C on heat shock protein and metabolic genes expression’, Animal, 14: 360-67.