ESR5: The role of neonatal supplementation of glutamine in modulating growth, gut functional development, and antioxidative status in low birthweight piglets
Background
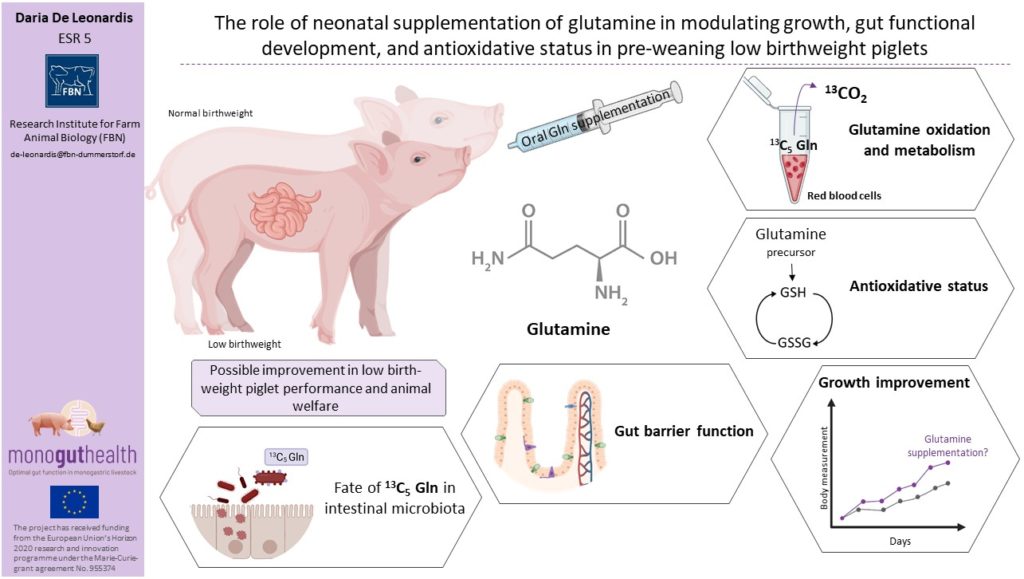
Maintaining high production rates to respond to the consumers’ demand for pork has led to genetic selection for increased litter size resulting in decreased piglet birthweight (BtW) and/or intrauterine growth restriction (IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin…) due to exceeding of uterine capacity. Low BtWPiglets weighing between 0.8 and 1.2 kg. (LBWPiglets weighing between 0.8 and 1.2 kg.) and IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… piglets have lower energy reserves and delayed access to colostrumThe first form of milk produced by sows immediately following birth of piglets. Colostrum has an esp… intake immediately after birth, and thus, tailored nutritional solution for those pigs might optimize gut health and development as well as growth performance. GlutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine… (Gln) is a major metabolic substrate for dividing cells, such as enterocytes, but has also a role in gene expressionA process by which information from a gene is used for protein synthesis. Ultimately this whole proc… regulation and cell signaling pathways. GlutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine… has been shown to be beneficial in intestinal maturation, gut oxidative and inflammatory prevention in weaned piglets.
Objectives
In contrast to earlier research where glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... was supplemented to weaned piglets, a novel paradigm of neonatalRelating to newborn individual. oral administration of glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... is employed to explore if glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... plays a regulating role in gut development in the pre-weaning period.
Investigate whether early postnatal oral glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... supplementation affects piglet growth and development, intestinal epithelium characteristics, in LBWPiglets weighing between 0.8 and 1.2 kg. and normal BtW piglets differently.
Study whether neonatalRelating to newborn individual. oral glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... supplementation affects the metabolic profile and the glutathione system, especially in LBWPiglets weighing between 0.8 and 1.2 kg. compared with normal BtW piglets.
Investigate if neonatalRelating to newborn individual. glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... supplementation influences the intestinal barrier function in vivo, the tight junction proteins and Gln incorporation in intestinal microbial protein.
Methods
High Performance Liquid ChromatographyA technique used to separate, identify and quantify components in a mixture; sample in the liquid (m... (HPLC) and automatic enzymatic analyser will be applied to determine the metabolites and amino acid concentration from plasma, intestine and liver.
Cell size (protein:DNA ratio), protein synthetic efficiency (protein:RNA ratio) and protein synthetic capacity (RNA:DNA ratio) will be determined in intestinal tissues.
Cellular proliferation and fractional protein synthesis rate will be measured using 5-bromo-2-deoxy-uridine and 2H5-phenylalanine application, respectively.
Gas-chromatography/mass spectrometry (GC/MS) and Isotope ratio MS analysis will be used to determine 2H5-phenylalanine abundance in tissue proteins and plasma as well as the oxidation of Gln and glucose and its metabolic conversion in amino acids, respectively.
Redox status will be determined using the reduced glutathione to oxidized glutathione ratio (GSH/GSSG), identified by HPLCA technique used to separate, identify and quantify components in a mixture; sample in the liquid (m... in red blood cells and intestinal tissue.
Transcripts related to intestinal functions will be analysed by qPCR. To evaluate intestinal barrier function, intestinal tissue will be collected for tight junction proteins analysis.
Intestinal barrier function will be evaluated with a sugar test, and intestinal tissue will be collected for the analysis of tight junction proteins abundance.
Expected results
Provide proof of concept and validated results that enteral glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine... supplements during the neonatalRelating to newborn individual. phase beneficially affect growth and gut health in pre-weaning LBWPiglets weighing between 0.8 and 1.2 kg. piglets (D1.3, D1.6, D2.7, D2.8, D2.10, D3.4).
Planned secondments
- At: WBF-A (1 mo); Learn how to identify IUGR piglets using Machine Learning Model.
- At: Kaesler Nutrition (2 mo); Training in scientific marketing for livestock nutrition and training in preparing technical documents for customers;
- At: Chemovator (1 mo); Training on literature search, scientific marketing of feed aditives;
Enrolment in Doctoral degree:
ESR5 will be enrolled at the Faculty of Veterinary Medicine, Free University of Berlin.
Supervisors
Cornelia Metges (FBN), Giuseppe Bee (Agroscope)