ESR blog
The number of piglets produced per sow per year is a key indicator of the production efficiency in modern sow production (Zhang et al., 2019). Most of the breeding programs focus on increasing litter survivability and consequently, the number of weaned piglets per litter (Hansen et al., 2018).
The increase in litter sizes observed in modern pig breeds has led to a significantly lower mean birth weight and increased the percentage of piglets born undersized and exposed to different degrees of intrauterine growth restriction (IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin…) (Hansen et al., 2018, Amdi et al., 2013). IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… is defined as the impaired development of the mammalian fetus or its organs during gestationA period of time between conception and birth. During this time, the fetuses (unborn piglets) grow a… (Wu et al., 2006). Low birth weight and IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… piglets usually show high morbidity and mortality, gut dysfunction, low efficiency of nutrient utilization, stunted growth and poor carcass quality (Wu et al. 2006). Several studies reported that pigs with a low birth weight have a decreased percentage of muscle and an increased percentage of body fat at slaughter (Gondret et al., 2006; Zhang et al., 2022; Krueger et al., 2014). Additionally, they require more days to reach the slaughter weight compared to their normal littermates (Gondret et al., 2005; Liu et al., 2015).
For these reasons, they represent a major economic loss for the swine producers and the development of breeding and management strategies to reduce their frequency is of great importance in pig production. Various solutions have been proposed by researchers to improve the vitality of low birth weight pigs and increase their survival rate.
Targeted dietary intervention in low birth weight piglets
Oral supplementation with colostrumThe first form of milk produced by sows immediately following birth of piglets. Colostrum has an esp… positively affected the growth performance of newborn piglets (Muns et al., 2014). Functional amino acids like glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine… or arginine have been reported to improve growth performance (glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine…, arginine), milk intake (glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine…), lipid metabolismChemical processes that occur within a living organism in order to maintain life. (glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine…) and intestinal function (arginine) in low birth weight pigs (Li et al., 2022; Zheng et al., 2018). Furthermore, supplementing L-carnitine from day 7 to day 27 improved skeletal myofiber formation and activated muscle hypertrophy of low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg. (Loesel et al., 2009).
Not all the (amino acid) supplementation strategies are beneficial for low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg.. For instance, leucine positively affected the growth performance of normal birth weight piglets whereas the same amino acid had a negative effect on the low birth weight ones (Ji et al, 2022).
Dietary interventions in sows
Dietary intervention in sows is another way to face the problem of low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg.. Indeed, even if the nutrient supply based on feeding recommendations like NRC (1998) is covered, adding specific nutrients can be useful for gestating sows as this could affect the offspring.
For example, adding arginine and its precursor glutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine… can be beneficial as they are amino acids involved in fetal growth (Wu et al., 2011). Berard and Bee (2010) focused their research on the use of arginine. L-arginine supplementation has been shown to improve the primary phase of myofiber formation. As mentioned in the introduction, L-arginine could be used to improve poor carcass quality in low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg.. Another amino acid, L-carnitine, has also been reported to affect protein and lipid metabolismChemical processes that occur within a living organism in order to maintain life. , which positively improved the body weight of newborn pigs (Musser et al., 1999). Lactoferrin supplementation in gestationA period of time between conception and birth. During this time, the fetuses (unborn piglets) grow a… diets of gilts increased the birth weight and decreased the number of dead and IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… piglets (Jahan et al., 2017).
The review written by Brown et al. (2011) evaluated metabolismChemical processes that occur within a living organism in order to maintain life. and amino acid transfer from the maternal circulation to the fetus across the placenta and deficit during IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… conditions. An evaluation of the impact of the most studied amino acids such as arginine, taurine and leucine has been made in this review. The conclusion was that due to some adverse fetal outcomes during some studies, a full understanding of the mechanism by which additional amino acids are transferred to the fetus warrants further knowledge.
In addition to the amino acids, other components like high fibre supplementation during gestationA period of time between conception and birth. During this time, the fetuses (unborn piglets) grow a… have been evaluated as a possible solution to low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg.. High fibre in diets has been investigated to have an effect on offspring, piglets nursed by sows fed with high-fibre lactation diets showed a higher growth rate in the first week of life (Guillemet et al., 2007).
Genetic selection of the sows
A possible approach to deal with low birth weight/IUGR piglets is genetic selection, at the piglet or the maternal level (Rothschild and Ruvinsky, 2011). The studies of Matheson et al. (2012) and Luna et al. (2014) in sheep and rats, showed the existence of some traits at the offspring level, like the neonates’ vitality or vigour, which can be easily identified. These traits are heritable (0.40 ± 0.04) and could be used for a direct selection at the piglet level (Matheson et al., 2018). However, increasing evidence suggests that the intrauterine environment is a major determinant of fetal growth, despite the important role of the fetal genome in prenatal development and growth (Vallet et al., 2014). The main cause behind poor intrauterine growth is at the maternal level and it is represented by an insufficiency of the placenta in distributing enough nutrients and oxygen to the offspring (Cohen et al., 2015).
The proportion of IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin…/low birth weight piglets in a litter is somehow correlated to the uterine capacity, which can be defined as the ability of the uterus to maintain the appropriate development of some number of conceptuses (Vallet et al., 2014). This proportion could be used for selection at the maternal level (Matheson et al., 2018).
Interventions at the microbiota level to deal with low birthweight piglets
In the last decade, the interaction between intestinal health and microbiota on the growth of pigs has received increased attention from the swine industry and academia (Kim & Duarte, 2021). Understanding the roles of the intestinal microbiota and their interaction with the host is essential in feed formulation (Duarte & Kim, 2022). Moreover, the modulation of the intestinal microbiota can lead to immediate and long-term effects on the intestinal health of pigs (Jang et al., 2020; Schokker et al., 2015). Early-life microbial exposure is potentially an effective intervention strategy for modulating the health and metabolismChemical processes that occur within a living organism in order to maintain life. of the host (Li et al., 2018). At the same time due to the increase of low birthweight piglets in today’s swine industry, new strategies to promote growth and intestinal function in neonates with IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… are urgently needed (Hu et al., 2017).
It is known that gut colonization by bacteria and the fermentation activity of the resulting intestinal microbiota are altered in neonates with low birthweight or IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin…, as compared with normal neonates (D’Inca et al., 2010; Huang et al., 2019). Studies show that IUGRIntra-uterine growth retardation, is defined as the impaired growth of the fetus or its organs durin… piglets had a decreased alpha diversity in the jejunum microbiota at 7 and 21 days of age, with a lower abundance of Bacteroidetes and Bacteroides in the jejunum. In contrast, they had higher abundances of Proteobacteria and Escherichia-Pasteurella in the ileum (Zhang et al., 2019).
This microbiota can be altered to beneficiate the abundance of certain beneficial species through the diet. For example, a supplementation on a diet with flaxseed oil increased specific phylum (Actinobacteria) and genera (Blautia and Bifidobacterium) that are beneficial for the gut health of the piglets (Che et al., 2019). Another option is to investigate the effect of the industrial processing of the food on the microbiota and oxidative status of the low birthweight piglets (Elmhiri et al., 2016).
However, what if we could alter the gut microbiota using bacteria?
Many studies have investigated the alteration of pig gut microbiota using Bacillus subtilis (Hu et al., 2017; Poulsen et al., 2018; Yun et al., 2021). B. subtilis is a Gram-positive facultative aerobic bacteria (Yun et al., 2021) capable of creating an anaerobic environment that supports the growth of beneficial bacteria such as Lactobacillus and Bifidobacteria by consuming oxygen in the intestine (Han et al., 2012). In other words, it can alter gut bacterial diversity by decreasing harmful bacteria and increasing beneficial bacteria (Han et al., 2012)
Another option is the use of Faecal Microbiota Transplantation (FMT), this method is gaining some interest in the pork industry as a method for establishing an appropriate microbiota in young piglets (Nowland et al., 2022). However, the results of the studies using this technique are contradictory, with some observing improvements in gut health while others observed a negative effect or no effect at all (Cheng et al., 2019; McCormack Ursula et al., 2018; Nowland et al., 2022).
Conclusions
Low birth piglets are a common productivity issue in nowadays swine industry, from an economic and welfare point of view. The probability of mortality in pigs is higher in low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg. and this is a multifactorial challenge that attracts the attention of industry, academia, and farmer in search of better solutions.
The survivability of the piglets can be improved using nutritional and/or genetic strategies, for example through gut health improvement and microbiota manipulation of the piglets or by using litter size variation in the sows for breeding programs.
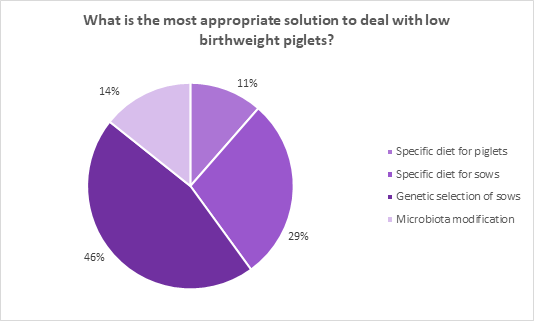
In the poll published on the MonoGutHealth LinkedIn page and Twitter page people voted between four answers to the question “What is the most appropriate solution to deal with low birthweight piglets?” Results show that 46% of the voters thought that genetic modification of the sows was the solution to the problem, while 29% thought that dietary intervention in the sow could overcome this challenge. Leaving a scarce 14 and 11% that think that a microbiota modification and a specific diet for piglets is the right answer (respectively) to the question.
References
Amdi, C., Krogh, U., Flummer, C., Oksbjerg, N., Hansen, C. F., & Theil, P. K. (2013). Intrauterine growth restricted piglets defined by their head shape ingest insufficient amounts of colostrumThe first form of milk produced by sows immediately following birth of piglets. Colostrum has an esp…. Journal of Animal Science, 91(12), 5605-5613. https://doi.org/10.2527/jas.2013-6824
Bérard J, Bee G. Effects of dietary l-arginine supplementation to gilts during early gestationA period of time between conception and birth. During this time, the fetuses (unborn piglets) grow a… on foetal survival, growth and myofiber formation. Animal. 2010;4(10):1680-1687. doi:10.1017/S1751731110000881
Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed). 2011;3(2):428-444. Published 2011 Jan 1. https://doi.org/10.2741/s162
Che, L., Zhou, Q., Liu, Y., Hu, L., Peng, X., Wu, C., Zhang, R., Tang, J., Wu, F., Fang, Z., Lin, Y., Xu, S., Feng, B., Li, J., Jiang, P., Wu, D., & Chen, D. (2019). Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiomeThe diverse consortium of bacteria, archaea, fungi, protozoa, viruses, and their collective genome f… and fatty acid profile in pigs with intrauterine growth retardation. Food Funct, 10(12), 8149-8160. https://doi.org/10.1039/c9fo01877h
Cheng, C. S., Wei, H. K., Wang, P., Yu, H. C., Zhang, X. M., Jiang, S. W., & Peng, J. (2019). Early intervention with faecal microbiota transplantation: an effective means to improve growth performance and the intestinal development of suckling piglets. Animal, 13(3), 533-541. https://doi.org/https://doi.org/10.1017/S1751731118001611
De Vos M, Che L, Huygelen V, et al. Nutritional interventions to prevent and rear low-birthweight piglets. J Anim Physiol Anim Nutr (Berl). 2014;98(4):609-619. https://doi.org/10.1111/jpn.12133
D’Inca, R., Kloareg, M., Gras-Le Guen, C., & Le Huërou-Luron, I. (2010). Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatalRelating to newborn individual. pigs. J Nutr, 140(5), 925-931. https://doi.org/10.3945/jn.109.116822
Duarte, M. E., & Kim, S. W. (2022). Intestinal microbiota and its interaction to intestinal health in nursery pigs. Animal Nutrition, 8(1), 169-184. https://doi.org/https://doi.org/10.1016/j.aninu.2021.05.001
Elmhiri, G., Hamoudi, D., Dou, S., Bahi-Jaber, N., Reygnier, J., Larcher, T., Firmin, S., & Abdennebi-Najar, L. (2016). Antioxidant properties of formula derived Maillard reaction products in colons of intrauterine growth restricted pigs. Food Funct, 7(6), 2582-2590. https://doi.org/10.1039/c5fo01551k
Gondret, F., Lefaucheur, L., Juin, H., Louveau, I., & Lebret, B. (2006). Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. Journal of animal science, 84(1), 93-103. https://doi.org/10.2527/2006.84193x
Han, G.-Q., Xiang, Z.-T., Yu, B., Chen, D.-W., Qi, H.-W., Mao, X.-B., Chen, H., Mao, Q., & Huang, Z.-Q. (2012). Effects of different starch sources on Bacillus spp. in intestinal tract and expression of intestinal development related genes of weanling piglets. Molecular Biology Reports, 39(2), 1869-1876. https://doi.org/10.1007/s11033-011-0932-x
Hansen, C. F., Hales, J., Amdi, C., & Moustsen, V. A. (2018). Intrauterine growth-restricted piglets defined by their head shape have impaired survival and growth during the suckling period. Animal Production Science, 59(6), 1056-1062. https://doi.org/10.1071/AN17581
Hu, L., Peng, X., Chen, H., Yan, C., Liu, Y., Xu, Q., Fang, Z., Lin, Y., Xu, S., Feng, B., Li, J., Wu, D., & Che, L. (2017). Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr, 56(4), 1753-1765. https://doi.org/10.1007/s00394-016-1223-z
Huang, S., Li, N., Liu, C., Li, T., Wang, W., Jiang, L., Li, Z., Han, D., Tao, S., & Wang, J. (2019). Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolomeDescribes the complete set of all low-weight molecules that can be quantified in a biological sample… in intrauterine growth restricted piglets during the first 12 hours after birth. J Microbiol, 57(9), 748-758. https://doi.org/10.1007/s12275-019-8690-x
Jahan M, Kracht S, Ho Y, et al. Dietary lactoferrin supplementation to gilts during gestationA period of time between conception and birth. During this time, the fetuses (unborn piglets) grow a… and lactation improves pig production and immunity. PLoS One. 2017;12(10):e0185817. Published 2017 Oct 12. https://doi.org/10.1371/journal.pone.0185817
Jang, K. B., Purvis, J. M., & Kim, S. W. (2020). Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiomeThe diverse consortium of bacteria, archaea, fungi, protozoa, viruses, and their collective genome f… of offspring. J Anim Sci, 98(8), skaa227. https://doi.org/10.1093/jas/skaa227
Ji Y, Sun Y, Liu N, et al. L-leucine supplementation reduces growth performance accompanied by changed profiles of plasma amino acids and expression of jejunal amino acid transporters in breast-fed intra-uterine growth-retarded piglets [published online ahead of print, 2022 Sep 1]. Br J Nutr. 2022;1-33. https://doi.org/10.1017/S0007114522002823
Kim, S. W., & Duarte, M. E. (2021). Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Animal bioscience, 34(3), 338-344. https://doi.org/10.5713/ab.21.0010
Li Z, Sciascia QL, Görs S, et al. GlutamineAn amino acid used for biosynthesis of proteins. One of the functions known is the role of glutamine… supplementation moderately affects growth, plasma metabolite and free amino acid patterns in neonatalRelating to newborn individual. low birth weight pigletsPiglets weighing between 0.8 and 1.2 kg. [published online ahead of print, 2022 Feb 11]. Br J Nutr. 2022;1-11. https://doi.org/10.1017/S0007114522000459
Li, N., Huang, S., Jiang, L., Wang, W., Li, T., Zuo, B., Li, Z., & Wang, J. (2018). Differences in the Gut Microbiota Establishment and MetabolomeDescribes the complete set of all low-weight molecules that can be quantified in a biological sample… Characteristics Between Low- and Normal-Birth-Weight Piglets During Early-Life [Original Research]. Frontiers in Microbiology, 9(1798). https://doi.org/10.3389/fmicb.2018.01798
Lin, G., Wang, X., Wu, G. et al. Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids 46, 1605–1623 (2014). https://doi.org/10.1007/s00726-014-1725-z
Liu, J. B., Yang, Y. K., & He, J. (2015). Intrauterine growth retardation increases lipid deposition in adipose tissue of pigs in response to high-fat/high energy diets. Livestock Science, 177, 95-102. https://doi.org/10.1016/j.livsci.2015.03.018
Lösel D, Kalbe C, Rehfeldt C. L-Carnitine supplementation during suckling intensifies the early postnatal skeletal myofiber formation in piglets of low birth weight. J Anim Sci. 2009;87(7):2216-2226. https://doi.org/10.2527/jas.2008-1662
Matheson, S. M., Walling, G. A., & Edwards, S. A. (2018). Genetic selection against intrauterine growth retardation in piglets: a problem at the piglet level with a solution at the sow level. Genetics Selection Evolution, 50(1), 1-11. https://doi.org/10.1186/s12711-018-0417-7
McCormack Ursula, M., Curião, T., Wilkinson, T., Metzler-Zebeli Barbara, U., Reyer, H., Ryan, T., Calderon-Diaz Julia, A., Crispie, F., Cotter Paul, D., Creevey Christopher, J., Gardiner Gillian, E., & Lawlor Peadar, G. (2018). Fecal Microbiota Transplantation in Gestating Sows and NeonatalRelating to newborn individual. Offspring Alters Lifetime Intestinal Microbiota and Growth in Offspring. mSystems, 3(3), e00134-00117. https://doi.org/10.1128/mSystems.00134-17
Muns R, Silva C, Manteca X, Gasa J. Effect of cross-fostering and oral supplementation with colostrums on performance of newborn piglets. J Anim Sci. 2014;92(3):1193-1199. https://doi.org/10.2527/jas.2013-6858
Musser RE, Goodband RD, Tokach MD, et al. Effects of L-carnitine fed during gestationA period of time between conception and birth. During this time, the fetuses (unborn piglets) grow a… and lactation on sow and litter performance. J Anim Sci. 1999;77(12):3289-3295. doi:10.2527/1999.77123289x
National Research Council (NRC) 1998. Nutrient requirements of swine. Natl. Acad. Press, Washington, D.C.
Nowland, T. L., Kirkwood, R. N., & Pluske, J. R. (2022). Review: Can early-life establishment of the piglet intestinal microbiota influence production outcomes? Animal, 16, 100368. https://doi.org/https://doi.org/10.1016/j.animal.2021.100368
Poulsen, A.-S. R., Jonge, N. d., Nielsen, J. L., Højberg, O., Lauridsen, C., Cutting, S. M., & Canibe, N. (2018). Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS One, 13(11), e0207382. https://doi.org/10.1371/journal.pone.0207382
Rothschild, M. F., & Ruvinsky, A. (2011). The genetics of the pig. CABI.
Schokker, D., Zhang, J., Vastenhouw, S. A., Heilig, H. G. H. J., Smidt, H., Rebel, J. M. J., & Smits, M. A. (2015). Long-Lasting Effects of Early-Life Antibiotic Treatment and Routine Animal Handling on Gut Microbiota Composition and Immune System in Pigs. PLoS One, 10(2), e0116523. https://doi.org/10.1371/journal.pone.0116523
Vallet, J. L., McNeel, A. K., Miles, J. R., & Freking, B. A. (2014). Placental accommodations for transport and metabolismChemical processes that occur within a living organism in order to maintain life. during intra-uterine crowding in pigs. Journal of animal science and biotechnology, 5(1), 1-14. https://doi.org/10.1186/2049-1891-5-55
Wu, G., Bazer, F. W., Wallace, J. M., & Spencer, T. E. (2006). Board-invited review: intrauterine growth retardation: implications for the animal sciences. Journal of animal science, 84(9), 2316-2337. https://doi.org/10.2527/jas.2006-156
Yun, Y., Ji, S., Yu, G., Jia, P., Niu, Y., Zhang, H., Zhang, X., Wang, T., & Zhang, L. (2021). Effects of Bacillus subtilis on jejunal integrity, redox status, and microbial composition of intrauterine growth restriction suckling piglets. J Anim Sci, 99(10). https://doi.org/10.1093/jas/skab255
Zhang, J., Yan, E., Zhang, L., Wang, T., & Wang, C. (2022). Curcumin reduces oxidative stress and fat deposition in longissimus dorsi muscle of intrauterine growth‐retarded finishing pigs. Animal Science Journal, 93(1), e13741. https://doi.org/10.1111/asj.13741
Zhang, W., Ma, C., Xie, P., Zhu, Q., Wang, X., Yin, Y., & Kong, X. (2019). Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. J Appl Microbiol, 127(2), 354-369. Guillemet R, Hamard A, Quesnel H, et al. Dietary fibre for gestating sows: effects on parturition progress, behaviour, litter and sow performance. Animal. 2007;1(6):872-880. https://doi.org/10.1111/jam.14304
Zheng P, Song Y, Tian Y, et al. Dietary Arginine Supplementation Affects Intestinal Function by Enhancing Antioxidant Capacity of a Nitric Oxide-Independent Pathway in Low-Birth-Weight Piglets. J Nutr. 2018;148(11):1751-1759. https://doi.org/10.1093/jn/nxy198